Abstract
BACKGROUND: Tyrosine kinase inhibitors (TKIs) are the standard of care for adult and pediatric patients (pts) with chronic myeloid leukemia in chronic phase (CML-CP). In adults, 5 TKIs are approved: imatinib, dasatinib, nilotinib, bosutinib and ponatinib. In the pediatric population, however, only imatinib, dasatinib and nilotinib are approved. Pediatric pts have similar cytogenetic and molecular response rates to first-line (1L) imatinib and 1L second-generation TKIs nilotinib and dasatinib as adults. While the safety profiles for TKIs are similar in adult and pediatric pts, growth retardation has been reported specifically in the pediatric population. Therefore, safer and more efficacious options are needed for pediatric pts. Asciminib is an investigational drug that inhibits BCR-ABL1 by specifically targeting the ABL myristoyl pocket (STAMP inhibitor). Asciminib has shown promising efficacy and safety in adults with CML-CP in phase I-III trials, given without food for 1 hour prior and 2 hours after taking asciminib (fasted). In the phase I asciminib monotherapy trial in heavily pretreated adult pts, 48% and 28% without and with T315I mutations, respectively, achieved or maintained major molecular response (MMR) by 12 months. In the phase III ASCEMBL trial for adults with CML-CP who are resistant to or intolerant of ≥2 TKIs, 25.5% of pts on asciminib vs 13.2% of pts on bosutinib achieved MMR at 24 weeks. Here we present the phase Ib/II trial evaluating asciminib monotherapy in pediatric pts.
OBJECTIVE: The primary objectives of this study are to characterize the pharmacokinetic (PK) profile of asciminib in pediatric pts and identify a pediatric formulation dose (taken with food) leading to an asciminib exposure comparable to that of 40 mg twice daily (BID) in adult pts (fasted).
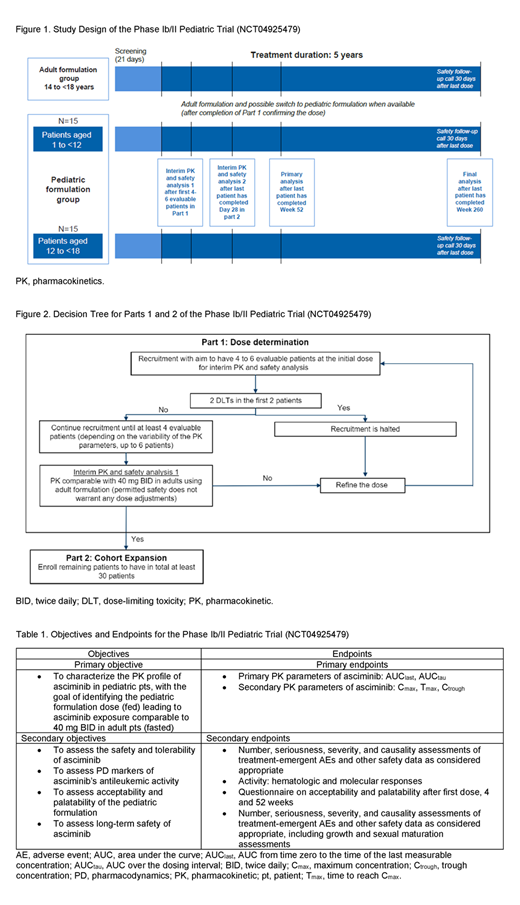
DESIGN: This is a multicenter, open-label, phase Ib/II study (NCT04925479, Figure 1). Eligible pts have Philadelphia chromosome-positive CML-CP resistant to or intolerant of ≥1 prior TKI, do not bear T315I mutations and are aged ≥1 to <18 years. Adolescent pts aged ≥14 to <18 years and weighing ≥40 kg will be enrolled in the exploratory adult formulation group, receiving 40 mg asciminib BID in the fasted state until the pediatric formulation is available. There is no prespecified sample size for this group.
In part 1 (dose-determining cohort), 4 to 6 pts aged ≥1 to <18 years will receive the pediatric formulation (body weight-adjusted dose of asciminib minitablets taken with food) to obtain ≥4 participants evaluable for PK data. This evaluation will determine whether median exposure in pediatric pts is comparable to that of asciminib 40 mg BID in adult pts (fasted). Dose adjustments will be made if the minimum exposure is less than that of 20 mg BID in adults or higher than double the maximum exposure in adults at 40 mg BID. During the first 28 days after treatment initiation, safety will be assessed based on predefined dose-limiting toxicities.
In part 2 (expansion cohort), the body weight-adjusted dosing determined in part 1 will be used, and the total pts enrolled on pediatric formulation will be increased to 30, including those from part 1 (15 pts aged 1 to <12 years and 15 pts aged 12 to <18 years). Pts receiving the adult formulation will have the opportunity to switch to the pediatric formulation in part 2 but will not be counted toward the 30 pts enrolled in part 2. A second interim PK and safety analysis will be conducted after all pts in part 2 have completed 28 days of treatment (Figure 2).
The total duration of the study treatment period is 5 years. The primary analysis (primary PK endpoints, safety, and pharmacodynamics) will be assessed after all pts have been on study for 52 weeks or discontinued earlier. A final analysis will be completed after all pts have been on study for 5 years or discontinued earlier. Pts who discontinue the study early will be followed up for survival until the study is complete.
MAIN OUTCOMES MEASURES: The primary endpoints include PK parameters. Secondary endpoints include treatment-associated AEs, hematologic and molecular responses, and acceptability and palatability of asciminib (Table 1).
CONCLUSIONS: Data from this study will be used to support a strategy of full extrapolation from adult data to use in the pediatric setting.
This study is sponsored by Novartis.
Hijiya: Novartis: Consultancy; Stemline Therapeutics: Consultancy. Kapoor: Novartis: Current Employment, Current equity holder in publicly-traded company. Descamps: Novartis: Current Employment. Bayar: Novartis: Current Employment. Ramscar: Novartis: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal